ORDER DEVELOPMENT
MIPM has been developing and manufacturing medical devices since 1982. Our core expertise are MRI-compatible devices. We develop our products in accordance with DIN EN ISO 13485 and are a CE certified company.
The close cooperation between our customers, project management, developers and production team guarantees you detailed information about the current status of your project throughout the entire development process.
Together with our customers, we develop the product specifications, analyze market requirements and incorporate legal and normative requirements to create a detailed set of specifications, which then form the basis for the development.
Well-known manufacturers have been relying on the know-how of MIPM for many years. They work with us in new developments as well as changes to already launched series products.
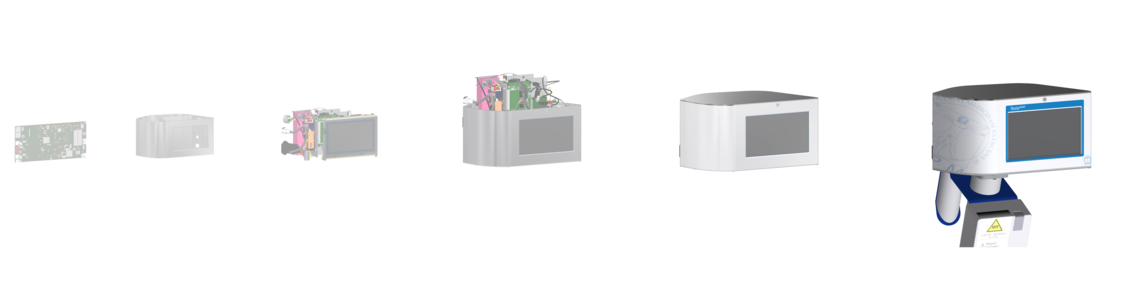
Support in all development stages
Stage 1: Definition of requirements
Development of a concept including feasibility (pre-selection and pre-testing) for devices and components.
Stage 2: Functional model
Development of detailed specifications. Functional models are designed and manufactured. Safety, functionality, efficiency, ergonomics and suitability for use are tested.
Stage 3: Prototype construction, verification and validation
Construction of production-quality prototypes. The product is verified and validated.
Stage 4: Zero-series production and validation
Design transfer (from development to production), the Zero-series is produced. Validation of the production process and regulatory requirements. Product release.
Stage 5: Series production and market surveillance
Series production and service. Activities for international approvals and registrations commence. The production process is re-validated. Market surveillance, including risk management and clinical assessment.



